Abstract
Background
High dose chemotherapy and autologous stem cell transplantation (ASCT) is a standard treatment approach for relapsed/refractory classical Hodgkin lymphoma (R/R HL). The combination of carmustine, etoposide, cytarabine, and melphalan (BEAM) is commonly used as the high-dose regimen, although there is no clear standard. Carmustine can induce life-threatening pneumonitis, or may be contraindicated in patients who have had bleomycin-related pneumonitis. The substitution of high-dose bendamustine at a dose of 200 mg/m2 for carmustine (Be-EAM) was shown to be safe and effective in a phase 1-2 study (Visani, Blood 2011), but Be-EAM has not been compared to other commonly used regimens.
Methods
Consecutive patients with R/R HL treated with Be-EAM and ASCT outside of clinical trials at the Hematology and Stem Cell Transplant Center, AORMN in Pesaro, Italy were identified using the PROMISE database. A separate cohort of 82 consecutive patients with R/R HL treated with BEAM and ASCT in Vancouver, Canada was identified using the BC Cancer Agency Centre for Lymphoid Cancer and the Leukemia/Bone Marrow Transplant Program of BC databases. Medical records were reviewed to obtain additional information.
BEAM patients were matched 2:1 to Be-EAM patients on the basis of two variables: primary refractory vs. relapsed disease after first-line therapy, and chemosensitivity to salvage therapy immediately prior to ASCT. Primary refractory HL was defined as progressive disease during or within 3 months of initial therapy. Characteristics, treatments, toxicity, and outcomes between both cohorts were compared.
Results
A total of 26 patients treated with Be-EAM were matched to 52 patients treated with BEAM. All patients were treated between 2009-2016, with the exception of 2 BEAM patients (2005, 2008). All patients failed initial treatment with standard combination chemotherapy regimens, most frequently ABVD, with or without radiation. Half of the patients in each cohort had primary refractory HL.
Be-EAM patients received ASCT after a median of 2 (range 1-5) lines of therapy for relapsed/refractory HL; regimens immediately prior to ASCT most commonly included ifosfamide, etoposide, and vinorelbine. BEAM patients were less pre-treated, with a median of 1 (range 0-2) line of therapy for relapsed/refractory HL; 50/52 received second-line chemotherapy with gemcitabine, dexamethasone, and cisplatin prior to ASCT, 1 received 2 lines, and 1 proceeded directly to ASCT without any preceding second-line chemotherapy.
Median age at ASCT was 34 (range 17-68), with no difference between groups (p=0.452). In each group, 73% patients were transplanted with chemo-sensitive disease, while the other 27% patients had active HL at the time of ASCT. Nine patients received post-ASCT radiation (1 Be-EAM, 8 BEAM), and 4 patients received maintenance brentuximab vedotin after BEAM.
Median time to absolute neutrophil recovery >0.5 x109/L was 10 days (range 7-17) Be-EAM vs. 11 days (range 6-20) BEAM. Median time to platelet recovery >20 x109/L was 12 days (range 6-22) Be-EAM and 10 days (6-20) BEAM. Immediately post-ASCT, there were 14 (54%) infections in patients treated with Be-EAM and 16 (31%) in patients treated with BEAM (p=0.083). Grade 3-4 mucositis was more common after Be-EAM than BEAM (35% vs. 10%, respectively, p=0.011). Two patients developed post-BEAM pneumonitis (1 also received consolidative mediastinal radiation).
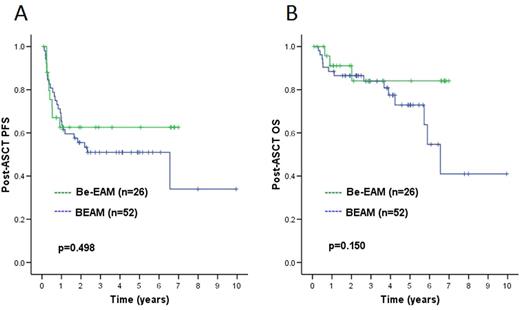
With a median post-ASCT follow-up of 2.1 years (range 10 months - 7 years) in Be-EAM patients and 3.9 years (range 1.1 - 16 years) in BEAM patients, the 2-year post-ASCT PFS was similar between both groups (63% Be-EAM vs. 56% BEAM, p=0.498), as shown in Figure 1A. Two-year post-ASCT OS was also similar between both groups (91% Be-EAM vs. 87% BEAM, p=0.150), as shown in Figure 1B, and the 4-year OS since diagnosis was also similar (87% Be-EAM vs. 88% BEAM, p=0.205). There have been 3 post-Be-EAM deaths (all HL) and 17 post-BEAM deaths (16 HL, 1 unrelated), with no treatment-related deaths.
Conclusions
Despite different patient and treatment characteristics between groups, particularly a significantly greater number of lines of therapy in patients undergoing Be-EAM, post-ASCT survival outcomes were similar between those receiving Be-EAM versus BEAM as high-dose therapy for R/R HL. These data require confirmation in prospective, randomized clinical trials.
Villa: Janssen: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Abbvie: Honoraria; Celgene: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Gerrie: Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Lundbeck: Honoraria; Roche: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Connors: Cephalon: Research Funding; Merck: Research Funding; Lilly: Research Funding; NanoString Technologies: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Bristol-Myers Squibb: Research Funding; F Hoffmann-La Roche: Research Funding; Amgen: Research Funding; Seattle Genetics: Research Funding; Takeda: Research Funding; Bayer Healthcare: Research Funding; NanoString Technologies, Amgen, Bayer, BMS, Cephalon, Roche, Genentech, Janssen, Lilly, Merck, Seattle Genetics, Takeda,: Research Funding. Savage: Roche: Research Funding; Seattle Genetics: Consultancy, Honoraria; Bristol-Myers Squibb: Honoraria; Merck: Honoraria; Celgene: Consultancy. Sutherland: Janssen: Honoraria. Sehn: Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Scott: Celgene: Consultancy, Honoraria; BCCA: Patents & Royalties: Patent describing molecular subtyping of DLBCL licensed to NanoString Technologies. Patent describing measurement of the proliferation signature in MCL.; Janssen: Consultancy, Honoraria. Isidori: Lundbeck: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal